The Laboratory of Chemistry of Synthetic and Natural Medicinal Substances (until May 20, 1994, the Laboratory of Organic Synthesis, from July 10, 2009, the Laboratory of Chemistry of Synthetic and Natural Medicinal Substances) was one of four laboratories at the time of establishing the Institute of Chemical Sciences in 1945. The first Head of the Laboratory was Irdan Nigmetovich Azerbayev, and since 1947 the Laboratory was headed by D.M. Sokolov, a war veteran, a student of Professor V. Chelintsev, later a Doctor of Chemical Sciences, Professor, Honored Scientist of the Kazakh SSR.
An important milestone in the Laboratory's research was a series of works on the synthesis and conformational analysis of bicyclic systems – the derivatives of ketodecahydroquinolines. D.V. Sokolov created a school of organic chemists, whose prominent representatives were G.S. Litvinenko, K.D. Praliyev, Zh.I. Issin, K.I. Khludneva, V.I. Artyukhin, V.V. Sosnova, B.T. Sydykov. As a result of systematic and targeted research, they developed simple and accessible methods for the preparation of a number of new decahydroquinoline-(4 or 5)-ones with different types of substitution in the heterocycle, and established general principles of the stereochemistry of the transformations of bicyclic amino ketones. In the 1970s, starting from cyclohexanone and vinyl acetylene, G.S. Litvinenko and his colleagues (K.I. Khludneva, D.G. Kim, E.G. Yalovenko, L.A. Issakova, T.K. Iskakova., L.A. Voronenko, O.V. Shiganakova) developed a shortened production process flowsheet for the local anesthetic Almakaine, recommended by the Pharmacological Committee of the USSR Ministry of Health for use in medicine, which made it possible to quickly and without additional costs obtain the target product in good yields. According to this technology, the first four stages were carried out in one reactor without isolating intermediate compounds. Herewith, the number of operations was reduced from 21 to 7, and the yield of 2-methyl-4-ketodecahydroquinoline increased from 40 up to 67%.
Technological findings received their brilliant development in the works of Hyasinth Stefanovich Litvinenko and his students in the 1980s - Ye.G. Yalovenko, N.Yu. Kuzmina, L.A. Voronenko, L.A. Issakova, T.K. Iskakova. As a result of the studies on the stereodirection of reactions at the carbonyl group in the carbo- and azacyclic series, they identified many stereochemical patterns, which led to the preparation of all eight theoretically possible racemic secondary alcohols based on four isomers of 2-methyl-4 -keto-trans-decahydroquinoline and four isomers. And the idea of using amidoketones as synthons was embodied in the development of methods for obtaining the previously inaccessible isomers of 2-methyldecahydroquinoline and 2,5-dimethylpiperidol-4. The scientists managed to obtain all four theoretically possible isomers of 2-methyl- and 10-methyl-5-oxidedecahydroquinolines, established a correlation between the steric direction of reduction and ethinylation of the keto group and the spatial structure of ketones.
It is also noteworthy that decahydroquinoline derivatives, synthesized by M.Z. Yessenaliyeva, S.A. Tarakov, O.T. Zhilkibayev and Ye.V. Fishchuk under the leadership of K.D. Praliyev, are of practical interest for medicine as antispasmodics, antihypoxants and local anesthetics. The results they obtained in this series of compounds was a significant contribution to the theoretical organic chemistry.
The wide range of scientific interests of Dmitriy Vassilyevich Sokolov included not only the issues of the chemistry of saturated nitrogen heterocycles. A natural alkaloid, ephedrine, produced at the Chimkent Chemical Plant, came to his attention. His meeting with Valentina Mikhailovna Kurilenko, the Head of the Laboratory of Pharmacology of the Novokuznetsk Chemical and Pharmaceutical Research Institute, one of the leading industry institutes of the USSR Ministry of Medical Industry, was a truly significant event. The result of this cooperation was the first original preparationg in the history of Kazakhstan - a new generation antidepressant Cephedrine, the derivative of ephedrine. Cephedrine is effective in the treatment of mentally ill patients with depression of various etiologies, significantly exceeding the effectiveness of the imported preparations melipramine and amitriptyline. For the creation, industrial development of production and introduction into medical practice of Cephedrine, D.V. Sokolov, Zh.I. Issin, K.D. Praliyev and Yu.G. Bossyakov, as part of the team of developers, were awarded the Prize of the Council of Ministers of the Kazakh SSR for 1986 in the field science and technology.
From 1981 to 1993, the Laboratory of Organic Synthesis was headed by one of the leading specialists of the Institute in the field of theoretical organic chemistry, fine organic and organoelement synthesis, Yu.G. Bossyakov, while simultaneously acting as the Deputy Director of the Institute for scientific work. The research carried out by Yu.G. Bossyakov with his fellow workers (D.G. Kim, A.P. Logunov, G.P. Revenko, O.V. Shiganakova, G.T. Maishinova), made it possible to create a new promising direction in the field of synthesis, stereochemistry and conformational analysis of C-functional organophosphorus compounds - phosphorinans and phosphabicyclodecanes, as well as boron, phosphorus and organosulfur compounds with practically useful properties.
As is known, one of the most pressing problems in medicine remains the fight against pain. Currently, intensive research is being conducted all over the world to search for highly effective analgesics, which have no or minimal narcotic potential, the so-called “ideal” analgesics. Since the beginning of the 70s of the last century, the systematic synthetic and stereochemical studies have been carried out in the Laboratory in a series of substituted piperidine derivatives to search for highly effective analgesics. The Head of the laboratory, academician of the National Academy of Sciences of the Republic of Kazakhstan, Kaldybai Dzhailovich Praliyev was the leader of these studies, first as a responsible executor, and then as a scientific supervisor. He headed the Laboratory since 1993.
These studies are carried out in two main directions: synthesis, stereochemistry and study of the chemical and spectral properties of new piperidine derivatives, on the one hand, and establishing the relationship between the chemical (spatial) structure of the resulting compounds and their pharmacological activity, on the other hand. As a result, the new scientific data have been obtained. which make it possible to draw important conclusions on the relationship between the fine chemical (stereochemical) structure of the substances and their reactivity, spectral and, most importantly, pharmacological properties. Among the N- and C-substituted 4-phenyl-4-propionyloxypiperidines, synthesized by the employees involved in the “analgesic” research area, a number of preparations have been discovered, exceeding morphine and promedol in the analgesic activity by 3-30 times, and by the breadth of pharmacological action - by 2-100 times, and in the doses, which bring pain relief without exhibiting a narcotic effect.
Zh.I. Issin, V.K. Yu, M.Z. Yessenaliyeva, N.A. Belikova, S.A. Tarakov, Ye.Ye. Fomichyova, G.S. Akhmetova, A.D. Nagimova, A. Kabdraissova, A. Abdildanova, Z. Orynbekova, A. Amantayeva, U. Kemelbekov, A.Yu. Ten, S.S. Zhumakova have made their contribution to the development of piperidine research area over the years.
It is also important that the high pharmacological activity of a number of compounds, obtained on the basis of 1-(2-ethoxyethyl)-4-oxopiperidine and 1-(2-ethoxyethyl)-4-ethynyl-4-hydroxyperidine, allows one to hope for a successful search for new highly active substances among their transformation products. In this regard, it is worth mentioning a great contribution to the development of this direction by Doctor of Chemical Sciences, Professor I.A. Poplavskaya, combining her rich experimental experience with piperidine research area. These studies are reflected in the works of K. Botbayeva, K. Bazhikova, Zh. Elizekova, M. Baimurzina and B. Nauryzova. Among the products of numerous modifications, they have synthesized the compounds with the hypotensive, analgesic, anesthetic, and antispasmodic activities.
Later, the idea of complicating the piperidine ring has been developed in the Laboratory (V.K. Yu, T.K. Iskakova, Ye.Ye. Fomichyova, N.A. Zhumanova, R.D. Mukhasheva, Zh.M. Zhaksibayeva, G.Ye. Berganayeva, A.E. Malmakova, A.B. Kaldybayeva). Polyheteropolycyclic compounds attract the attention of synthetic chemists primarily because of the prospects for creating new effective preparations with a high selective effect on their basis. In this regard, the research is being conducted to study the chemical behavior of piperidones-4 in the reaction of heterocyclization, involving a carbonyl group and alpha hydrogens. Among the synthesized derivatives of 3,7-diheter(N,N-;N,S-;N,O-)bicyclo[3.3.1]nonane, non-narcotic analgesics and an opiate antagonist at the level of nalaxone have been found for the first time.
The Laboratory staff have not been lost on another acute problem both for Kazakhstan and for the whole world, the problem of combating tuberculosis. As is known, the spread of tuberculosis in the world has acquired alarming proportions; in Kazakhstan, morbidity and mortality indices (165.2 and 24 per 100,000 population in 2003), according to the WHO data, classify the country as one of the ten countries with the highest prevalence of tuberculosis. The scale of the problem is growing with the spread of drug-resistant strains of M. tuberculosis. The search for new anti-tuberculosis preparations is pursuing the development of such preparations, which are active against sensitive and resistant strains of M. tuberculosis and have low toxicity. The research carried out by the team under the guidance of Doctor of Chemical Sciences, Professor L.A. Kayukova - I.S. Zhumadildayeva, A.L. Akhelova, M. Orazbayeva, G.P. Baitursynova, A.B. Uzakova and Ye. Yergaliyeva are aimed at searching for new tuberculostatics, active against sensitive, resistant strains and non-toxic. An in vitro screening for the antituberculosis activity of the new β-aminopropioamidoxime derivatives they synthesized, has revealed the preparations with the activity exceeding the activity of rifampicin on sensitive strains of M. tuberculosis by 2 to 100 times, and on resistant strains by 2 to 200 times. And as a result of animal testing, it has turned out that one compound has more favorable pathomorphological indicators against sensitive and multidrug-resistant strains of M. tuberculosis than the main tuberculostatics - rifampicin and isoniazid. An interesting point in the biological screening has been the discovery in the series of O-benzoyl-β-aminopropioamidoximes of the preparations with the antiarrhythmic and local anesthetic properties, which have been more active than those of lidocaine and etmozin. Among the derivatives of β-aminopropioamidoximes, the compounds have been found, which are non-narcotic analgesics with the anti-inflammatory activity, pronounced hypnotic and antipyretic activity, as well as antidiabetic activity, with the acceptable toxicological parameters.
The successes of the Laboratory would not be so impressive if not for the close and fruitful cooperation with the medical and biological organizations: Al-Farabi Kazakh National University, S.F. Asfendiyarov Kazakh National Medical University, Zhiyembayev Kazakh Research Institute of Plant Protection and Quarantine, Kazakh Research Institute of Veterinary Medicine, the South Kazakhstan Medical Academy (Shymkent) and Grodno Medical University (Belarus).
A large block of scientific research on LCSNMS has been carried out within the frames of the international scientific cooperation with the Oklahoma State University (Prof. Darrell K. Berlin, USA), the New York State University (Prof. John Welch, USA), the Syracuse Medical Center (Prof. Michael Sineman, USA) and the University of Ghent (Prof. Norbert De Kimpe, Belgium), Prof. Sh. Yasui, and has been supported by the grants from the International Scientific Foundations CRDF (USA), INTAS (European Union) and ISTC.
Today, LCSNMS is a leading center for training highly qualified specialists in the field of fine organic chemistry of biologically active preparations for the country – the representatives of the Kazakh-British Technical University, Al-Farabi Kazakh National University, Asfendiyarov Kazakh National Medical University, Abai Kazakh National Pedagogical University and Kazakh National Women's Teacher Training University successfully defend theses, master's theses and PhD theses.
New times dictate new conditions, but the Laboratory invariably remains at the forefront of the development of fine organic synthesis of biologically active substances, conducting research within the frames of the government grants.
The Fatherland highly appreciated the scientific success of the Laboratory. Academician of the National Academy of Sciences of the Republic of Kazakhstan K.D. Praliyev, Doctor of Chemical Sciences, Professor D.V. Sokolov (posthumously), Ph.D., Associate Professor V.K. Yu became the Winners of the State Prize of the Republic of Kazakhstan in the Field of Science, Technology and Education in 2003 for the search, creation, organization of the production and introduction into widespread medical practice of original domestic synthetic medicines, in particular the analgesic Procidol and the local anesthetic and antiarrhythmic Kazcaine.
Nowadays the Laboratory is headed by the Winner of the State Prize of the Republic of Kazakhstan, Doctor of Chemical Sciences, Full Professor Valentina Konstantinovna Yu.
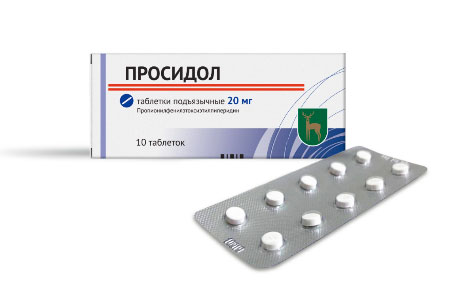
PROCIDOL — a highly effective analgesic, created jointly by the scientists and specialists of A.B. Bekturov Institute of Chemical Sciences and Novokuznetsk Chemical and Pharmaceutical Research Institute (the Russian Federation). Procidol is used for premedication; as an analgesic component of general anesthesia during surgical operations; postoperative pain relief; pain from burns; pain syndrome due to injuries; labor pain relief for women in childbirth with histosis; chronic pain syndrome in patients with occlusive lesions of the arterial system; chronic pain syndrome in cancer patients. The analgesic effect of Procidol occurs within 1-15 minutes and lasts 3-8 hours, in some cases up to 16 hours. When using Procidol, no depression of breathing or intestinal motility is observed.
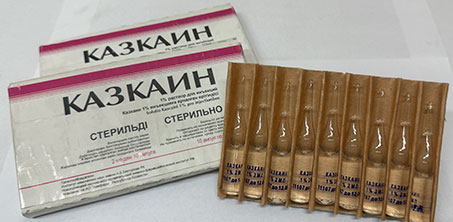
KAZCAINE — local anesthetic and antiarrhythmic, created by the joint efforts of the scientists and specialists of A.B. Bekturov Institute of Chemical Sciences, S.D. Asfendiyarov Kazakh National Medical University, and the Novokuznetsk Chemical and Pharmaceutical Research Institute. Kazcaine as a local anesthetic is recommended for infiltration and conduction anesthesia, which has low toxicity and a long-lasting effect. The preparation makes it possible to avoid many side effects of using central analgesics. Kazcaine as an antiarrhythmic agent is recommended for ventricular arrhythmias and ventricular fibrillation of various genesis, has a preventive and relieving effect.




